- Registration Service of Medical Devices
- CRC services Medical Device Business License Medical Device Registration Contract Research Organization ISO 13485 Quality System Certification GMP CE Certification Service FDA Registration (Filing) Software Integrity Verification Service Production process validation service ISO 15378 Medical Package System Certification ISO 13485 Internal Auditors Training Regulatory Services For Medical Devices
- Information security service of medical
- ISO 27001 Information Security Certification ISO 20000 Information Service Certification Information system grade protection filing Business Continuity Management Services Internal Auditor Training
- Medical Software Development
- Software of Good Supply Practice(GSP) Production System Development Customized software development
- CONTACT US
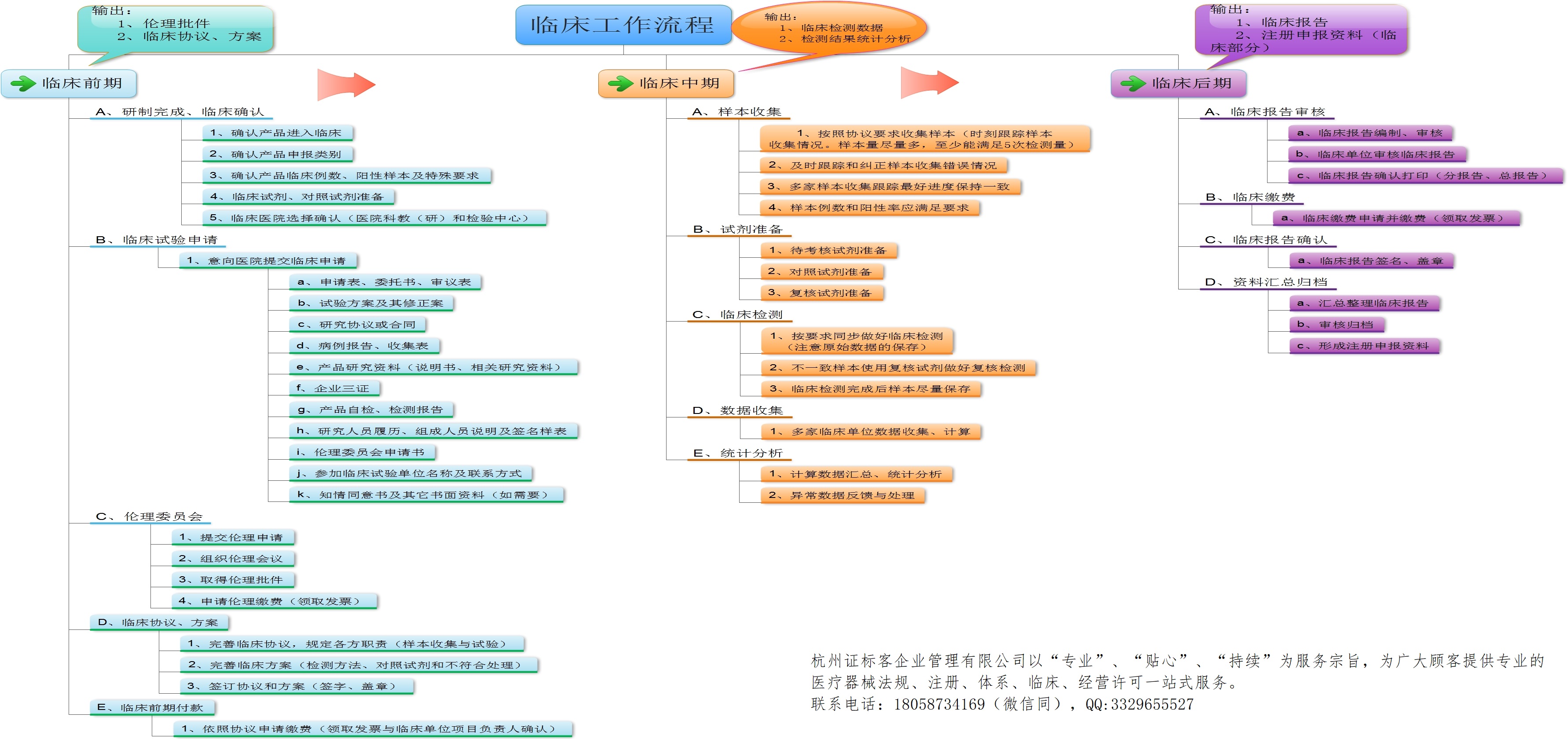
IVD clinical trial workflow chart
CREATE TIME:2018-07-09 16:15BROWSE TIMES:2324
IVD臨床試驗(yàn)工作流程圖,簡(jiǎn)要說(shuō)明了臨床試驗(yàn)的各個(gè)階段,各階段主要工作流程及要求。
PREVIOUS: NOTHING
NEXT: 醫(yī)療器械臨床服務(wù)流程
NEXT: 醫(yī)療器械臨床服務(wù)流程