- Registration Service of Medical Devices
- CRC services Medical Device Business License Medical Device Registration Contract Research Organization ISO 13485 Quality System Certification GMP CE Certification Service FDA Registration (Filing) Software Integrity Verification Service Production process validation service ISO 15378 Medical Package System Certification ISO 13485 Internal Auditors Training Regulatory Services For Medical Devices
- Information security service of medical
- ISO 27001 Information Security Certification ISO 20000 Information Service Certification Information system grade protection filing Business Continuity Management Services Internal Auditor Training
- Medical Software Development
- Software of Good Supply Practice(GSP) Production System Development Customized software development
- CONTACT US
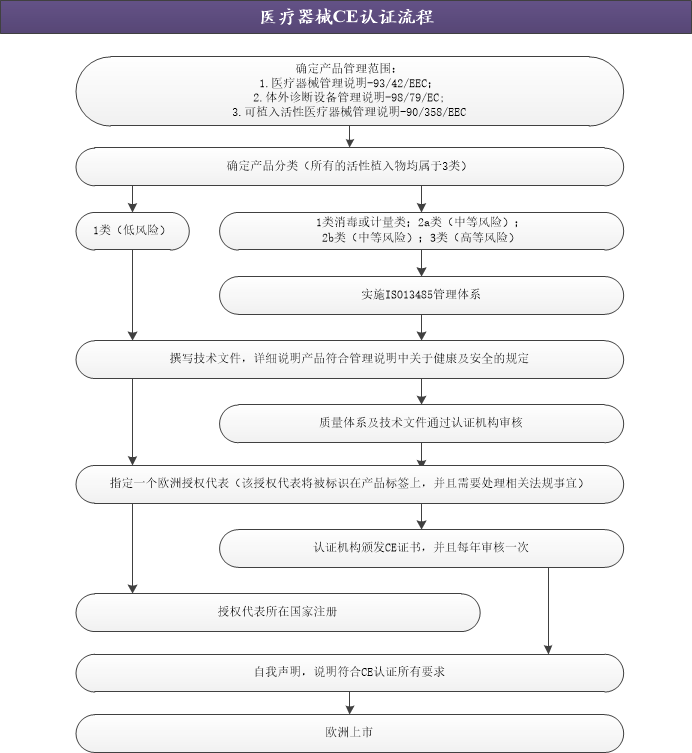
醫(yī)療器械CE認證流程
CREATE TIME:2018-06-29 11:34BROWSE TIMES:1786
“CE”標志是一種產(chǎn)品安全認證標志,被視為制造商打開并進入歐洲市場的通行證。在歐盟市場“CE”標志屬強制性認證標志,不論是歐盟內(nèi)部企業(yè)生產(chǎn)的醫(yī)療器械,還是其他國家生產(chǎn)的醫(yī)療器械,如果要在歐盟市場上自由流通,就必須加貼“CE”標志,以表明醫(yī)療器械分別符合歐盟《有源植入醫(yī)療器械指令》、《醫(yī)療器械指令》、《體外診斷器械指令》的基本要求。
PREVIOUS: NOTHING
NEXT: NOTHING
NEXT: NOTHING